Atoms Isotopes And Ions
Subject: Science
Grade: High school
Topic: Chemistry
Please LOG IN to download the presentation. Access is available to registered users only.
View More Content
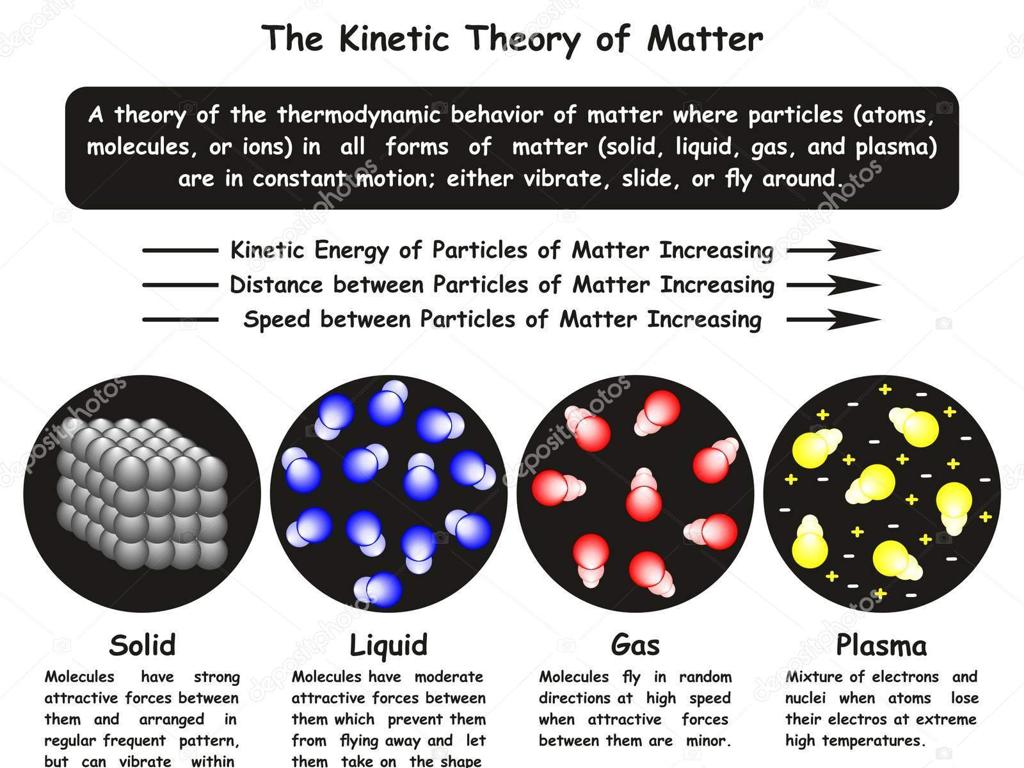
Atoms, Isotopes, and Ions: Building Blocks of Matter
– Atoms: The basic units of matter
– Consist of protons, neutrons, and electrons
– Isotopes: Atoms with varying neutrons
– Same element, different mass. E.g., Carbon-12 vs. Carbon-14
– Ions: Charged atoms or molecules
– Gain or lose electrons, resulting in positive or negative charge
– Their role in understanding chemistry
|
This slide introduces the fundamental concepts of atoms, isotopes, and ions, which are crucial for understanding the composition and behavior of matter in chemistry. Atoms are the smallest units that define the chemical elements and their properties. Isotopes are variations of the same element with different numbers of neutrons, leading to different atomic masses. Ions are atoms or molecules that have gained or lost electrons, acquiring a charge. These charged particles play a significant role in chemical reactions and bonding. The understanding of these concepts is essential for students as they form the basis for exploring more complex topics in chemistry. Encourage students to think about how these small particles make up the world around them and to ask questions about the material to deepen their comprehension.
Exploring the Atom
– Atom defined
– Smallest unit of matter, retains element’s properties
– Atom’s core structure
– Composed of protons, neutrons, and electrons
– Periodic table atom examples
– Hydrogen, Helium, Oxygen illustrate variety in atoms
– Significance of atomic structure
– Understanding leads to insights into chemical behavior
|
This slide introduces the fundamental concept of an atom, which is the basic building block of matter. Each atom is made up of a nucleus containing protons and neutrons, with electrons orbiting around it. The number of protons determines the element and its placement on the periodic table. Examples like Hydrogen (1 proton), Helium (2 protons), and Oxygen (8 protons) show the diversity of atomic structures. A firm grasp of atomic structure is crucial for students as it forms the basis for understanding chemical reactions, bonding, and properties of elements. Encourage students to explore the periodic table and relate the atomic structure to the element’s characteristics.
Isotopes Explained
– Defining isotopes
– Isotopes: same element, varying neutrons
– Atoms with identical protons but differing neutron counts
– Isotopes in the real world
– Carbon isotopes: Carbon-12, Carbon-13 used in dating
– Applications of isotopes
– Medical imaging, cancer treatment, and radiocarbon dating
|
Isotopes are variants of a particular chemical element which differ in neutron number, while retaining the same number of protons. This slide aims to clarify the concept of isotopes and how they are still the same element despite the difference in mass. Real-world examples include Carbon-12 and Carbon-13, which are crucial in archaeological dating methods like radiocarbon dating. Isotopes have significant applications in various fields such as medical imaging, where they are used in PET scans, and in cancer treatment through radiation therapy. Understanding isotopes is fundamental in high school chemistry, as it lays the groundwork for more advanced topics in nuclear chemistry and physics.
Ions and Their Charges
– Ions: Atoms with a charge
– An ion is an atom that has lost or gained electrons, resulting in a net charge.
– Cations vs Anions
– Cations are positively charged ions, Anions are negatively charged.
– Formation of Ions
– Ions form when atoms gain or lose electrons to achieve a full outer shell.
– Significance of Ions
– Ions are essential for electricity, signaling in the body, and chemical reactions.
|
This slide introduces the concept of ions, which are atoms or molecules that carry a net electrical charge. Students should understand that ions are formed when atoms lose or gain electrons, which can occur during chemical reactions or through the influence of an external force. The distinction between cations (positive charge) and anions (negative charge) is crucial, as it determines how ions will interact with each other. Emphasize the role of ions in various biological and physical processes, such as nerve impulse transmission and the formation of salts. Provide examples like Na+ and Cl- coming together to form table salt (NaCl). This foundational knowledge is important for understanding chemical bonding and reactions.
Atomic Mass and Atomic Number
– Finding the atomic number
– Atomic number (Z) represents the number of protons in the nucleus of an atom.
– Significance of atomic mass
– Atomic mass (A) reflects the atom’s total number of protons and neutrons.
– Calculating neutrons in an atom
– Subtract atomic number (Z) from atomic mass (A) to find the number of neutrons.
– Atomic number vs. mass number
|
This slide aims to explain the fundamental concepts of atomic mass and atomic number, which are crucial for understanding the structure of atoms. The atomic number is the number of protons in an atom’s nucleus and defines the element. Atomic mass, also known as mass number, is the combined count of an atom’s protons and neutrons, providing insight into the isotope of the element. By subtracting the atomic number from the atomic mass, students can calculate the number of neutrons, which is essential for understanding isotopes. Ensure students grasp that while the atomic number is unique to each element, atomic mass can vary due to the presence of isotopes.
Isotopic Notation in Chemistry
– Writing isotopes with notation
– Isotopes are written as X-A where X is the element symbol and A is the mass number.
– Decipher symbols and numbers
– The lower number (Z) is the atomic number, representing protons; the upper number (A) is the mass number, sum of protons and neutrons.
– Practice isotopic identification
– Given notation, determine element, atomic number, mass number, and number of neutrons.
– Mastery through examples
|
This slide introduces students to the concept of isotopic notation, a standardized way of representing different isotopes of an element. Isotopic notation includes the element’s symbol, atomic number, and mass number. The atomic number (Z) is located at the lower left of the element’s symbol, indicating the number of protons, which defines the element. The mass number (A) is at the upper left, representing the total number of protons and neutrons. Students will practice identifying isotopes by interpreting these notations and calculating the number of neutrons by subtracting the atomic number from the mass number. Provide several examples for the class to work through and encourage peer discussion to solidify understanding.
Ions in Chemical Reactions
– Ions’ role in reactions
– Ions, charged particles, are essential for forming compounds.
– Balancing ionic charges
– Equal positive and negative charges are necessary for stability.
– Common ionic compounds
– NaCl (table salt), CaCO3 (limestone), and HCl (hydrochloric acid).
– Uses of ionic compounds
– Salt in food, limestone in construction, acid for cleaning.
|
This slide aims to explain the significance of ions in chemical reactions, particularly in the formation of ionic compounds. Ions are atoms or molecules that have lost or gained electrons, resulting in a net charge. In chemical reactions, ions interact to form new substances, and the balance of charges is crucial for the stability of the compounds formed. Provide examples of common ionic compounds such as sodium chloride, calcium carbonate, and hydrochloric acid, and discuss their everyday uses. This will help students connect the concept of ions to tangible items and applications they are familiar with. Encourage students to think of other ionic compounds and their uses to reinforce the concept.
Class Activity: Building Atomic Models
– Construct atom models using materials
– Create isotopes with varying neutrons
– Isotopes have the same protons but different neutrons, e.g., Carbon-12 vs. Carbon-14
– Form ions by adding or removing electrons
– Ions form when atoms gain or lose electrons, resulting in a charge
– Discuss the significance of each model
|
This hands-on activity is designed to help students visualize and differentiate between atoms, isotopes, and ions. By using colored balls or marshmallows to represent protons, neutrons, and electrons, students can create physical models of these particles. Toothpicks or sticks will represent the bonds. The objective is to solidify the students’ understanding of atomic structure and the concept of isotopes and ions. For example, they can build a model of a neutral carbon atom, then create an isotope by adding or removing neutrons, and finally, create an ion by adding or removing electrons to give the atom a charge. Encourage students to discuss how the changes affect the properties of the atom. Possible activities include building models of common isotopes or simulating ion formation in salt (NaCl).
Atoms, Isotopes, and Ions: Summary & Applications
– Recap: Atoms and their structure
– Atoms are the basic units of matter, composed of protons, neutrons, and electrons.
– Isotopes and their significance
– Isotopes have the same number of protons but different neutrons, affecting atomic mass.
– Ions and their role in chemistry
– Ions are charged atoms, essential in forming compounds and conducting electricity.
– Real-world applications
– These concepts are crucial in fields like medicine, energy, and environmental science.
|
This slide aims to consolidate the students’ understanding of atoms, isotopes, and ions, emphasizing their importance in both theoretical chemistry and practical applications. Begin by summarizing the structure of atoms and how they form the building blocks of matter. Explain isotopes and their relevance, particularly in nuclear chemistry and medical imaging techniques. Discuss ions, their formation, and necessity in various chemical reactions and processes such as electrolysis. Highlight real-world applications to help students appreciate the relevance of these concepts beyond the classroom. Conclude with a Q&A session to address any remaining uncertainties and reinforce learning.